Novel Inhibitors of malarial aspartyl proteases, plasmepsin II and IV: In silico design and validation studies
Keywords:
anti-malarial drugs, molecular modeling, Molecular docking, heterocyclic drugs, protein bindersAbstract
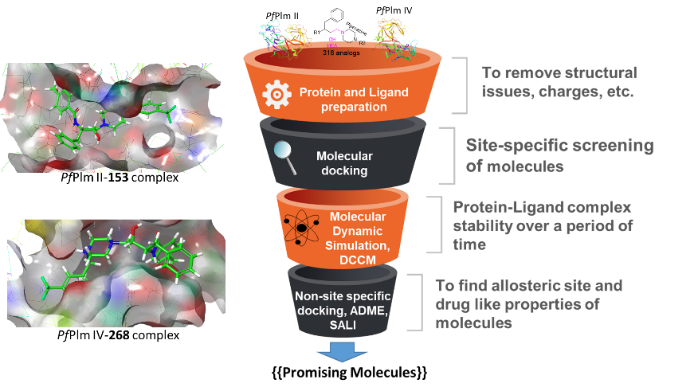
In the dire need of novel inhibitors of enzymes, computational approaches have significantly expedited the drug discovery process. Aspartic protease enzymes of Plasmodium falciparum such as plasmepsin II (PfPlm II) and plasmepsin IV (PfPlm IV) have been recognized as an attractive drug target for antimalarial drug discovery. In line with this, we performed high-throughput screening of 316 novel compounds based on validated pharmacophore i.e., hydroxyethylamine (HEA) and piperazine against both PfPlm II and PfPlm IV. The obtained hit compound-protein complexes were subjected for molecular dynamics (MD) simulations at 200ns and found stable. Thermodynamic energy calculated for the complexes also supported compound’s stability within the binding pocket of plasmepsins. The results of our study strongly support an immediate validation of the virtually screened hits in biological systems.



