Synthesis, anticancer activity and molecular docking study of (E)-4-(3,4-Dichlorophenyl)-2-((1,3-diphenyl-1H-pyrazol-4-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one derivatives
DOI:
https://doi.org/10.62110/sciencein.cbl.2024.v11.656Keywords:
Claisen-Schmidt condensation, Sertralone, pyrazole aldehyde, anticancer activity, Molecular docking, medicinal chemistry, heterocyclic drugsAbstract
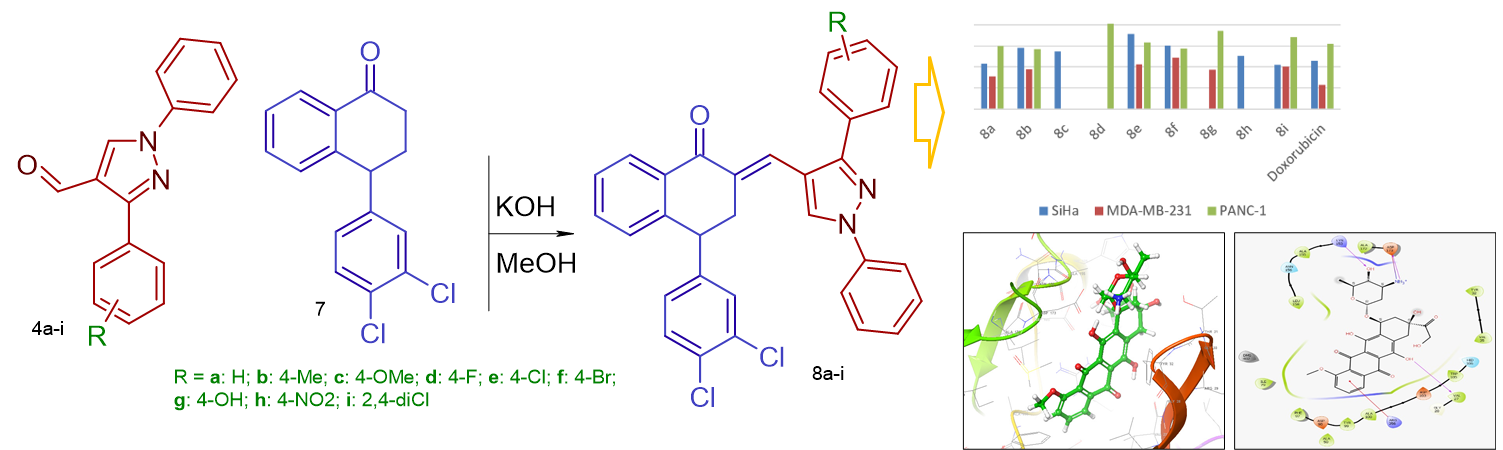
A novel series of (E)-4-(3,4-Dichlorophenyl)-2-((1,3-diphenyl-1H-pyrazol-4-yl)methylene)-3,4-dihydronaphthalen-1(2H)-one derivatives were synthesized by Claisen-Schmidt condensation of 4-(3,4-dichlorophenyl)-3,4-dihydronaphthalen-1(2H)-one and 1,3-diphenyl-1H-pyrazole-4-carbaldehyde. All the synthesised targets were evaluated for their cytotoxicity against a panel of three cancer cell lines (SiHa, MDA-MB-231 and PANC-1). Among the tested compounds many of them exhibited significant anticancer activity, the compound 8a was found to be the most promising analogue in this series with IC50 values of on tested three cancer cell lines.
URN:NBN:sciencein.cbl.2024.v11.656







