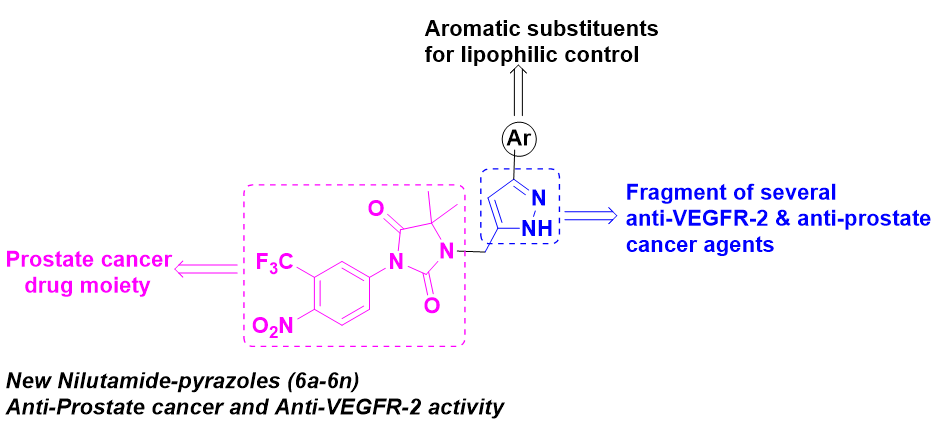
Synthesis of new Nilutamide-pyrazole derivatives as VEGFR-2 targeting anti-prostate cancer agents
DOI:
https://doi.org/10.62110/sciencein.cbl.2024.v11.668Keywords:
pyrazoleAbstract
Herein, we synthesized new Nilutamide-pyrazoles (6a-6n) via N-alkynylation and PdCl2(PPh3)2/CuI catalyzed tandem one-pot acyl-Sonogashira coupling followed by cyclo-condensation approaches. The in vitro anti-proliferative activity of these compounds against two human prostate cancer cell lines (PC-3 and DU-145) revealed that many of investigated compounds have shown better activity against PC-3 cell line as compared to DU-145 cell line. In particular, compounds 6d, 6f and 6m had higher activity against PC-3 than the standard drug 5-Fluoro Uracil (5-FU) with IC50 values <65 mM. As well, compound 6f displayed (IC50 = 39.1 mM) almost similar activity as 5-FU against DU-145 (IC50 = 38.5 mM). The in vitro VEGFR-2 inhibition studies revealed that compound 6f showed higher activity (IC50 = 26.1 nM) against VEGFR-2 than the standard drug Sorafenib (IC50 = 30 nM), whereas, compound 6d was shown comparable inhibition (IC50 = 30.6 nM) with the positive control. Finally, in silico molecular docking studies were described important binding interactions of most potent compounds 6d, 6f, 6m and Sorafenib with VEGFR-2 (pdb id 3VHE) and these compounds showed promising binding energies and inhibition constants than the Sorafenib.
URN:NBN:sciencein.cbl.2024.v11.668







