Anti-prostate cancer and anti-EGFR activities of new Nilutamide-isoxazole hybrids
Keywords:
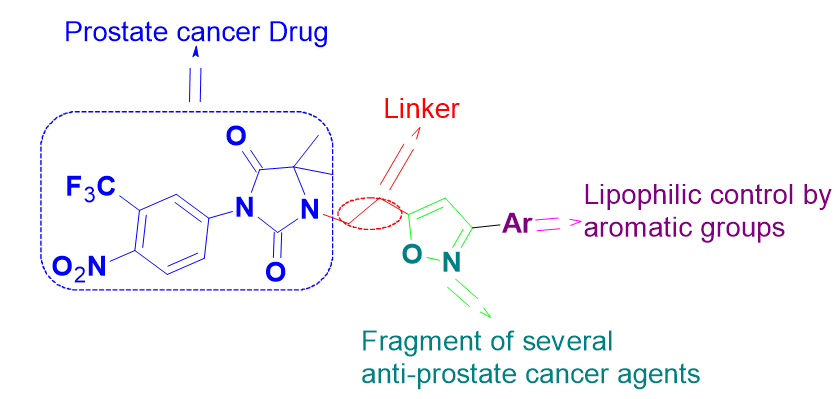
Nilutamide, Isoxazole, Prostate cancer, EGFR inhibition , Drug derivates, click chemistryAbstract
Herein, synthesis of new Nilutamide-isoxazoles (5a-5n) via Cu(I)-promoted one-pot reaction between 1-(but-3-yn-1-yl)-5,5-dimethyl-3-(4-nitro-3-(trifluoromethyl)phenyl)imidazolidine-2,4-dione (3) and several aldehydes (4a-4n) in benign aq. tbutanol as key approach has been reported. The in vitro growth inhibition activity of all these compounds revealed that the majority of compounds were more active against DU-145 in comparison to PC3. Particularly, compounds 5f, 5h and 5k showed greater activity against DU-145 than the standard drug 5-Fluoro Uracil with IC50 values <30 mM. whereas compound 5g showed comparable activity against DU-145 cell line with the positive control. The Epidermal growth factor receptor (EGFR) is well known to be expressed in DU-145 cancer cells, the most potent compounds 5f, 5h and 5k were then screened for their inhibitory potential against tyrosine kinase EGFR and found that compounds 5f and 5k showed remarkable inhibition with MIVs 93.4% and 91.3% respectively, while compound 5h displayed good inhibition (MIV = 84.6%) as compared to the Erlotinib.
URN:NBN:sciencein.cbl.2023.v10.542



