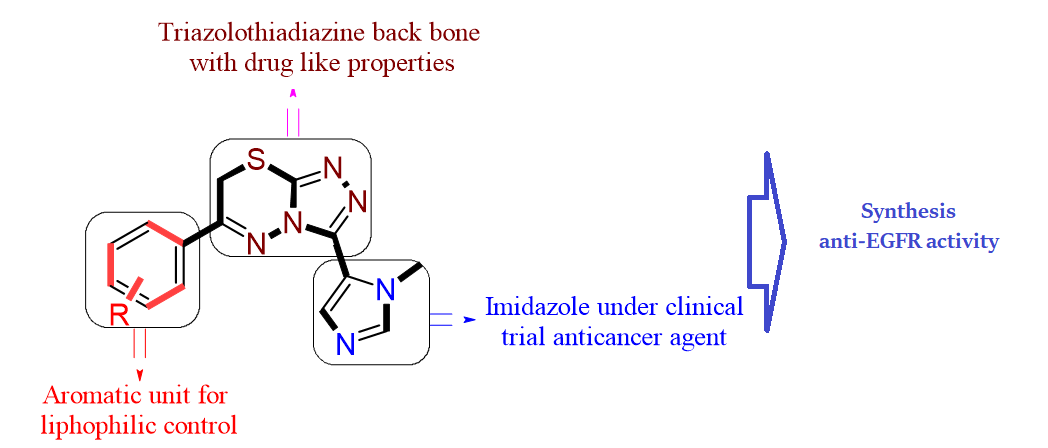
Synthesis of novel imidazolo-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine hybrids as in vitro EGFR inhibitors
Keywords:
Imidazole, 1,2,4-triazole, thiadiazine, anticancer activity, EGFR, EGFR inhibitory activityAbstract
The synthesis of new hybrid [1,2,4] triazolo [3,4-b][1,3,4]thiadiazine derivatives of imidazole (5a – 5m) and their structure determination using 1HNMR, 13CNMR and mass spectral analysis were described. The in vitro cytotoxic activity of the compounds (5a – 5m) against three human cancer cell lines like MCF-7 and MDA-MB-231 (breast), alveolar (A-549) revealed that the compounds 5c, 5d, 5f, 5g, and 5m have shown greater activity against breast cancer cell lines than the remaining compounds. Compounds 5d and 5f have shown equipotent activity compared to the standard. In vitro tyrosine kinase EGFR inhibition assay for the same more potent compounds (5c, 5d, 5f, 5g, and 5m) revealed that 5f has more potent inhibiting power with an IC50 value of 0.412±0.05 μM and 5d has equipotent inhibiting power with an IC50 value of 0.436±0.07 μM compared to erlotinib (IC50=0.423±0.03).
URN:NBN:sciencein.cbl.2023.v10.548



