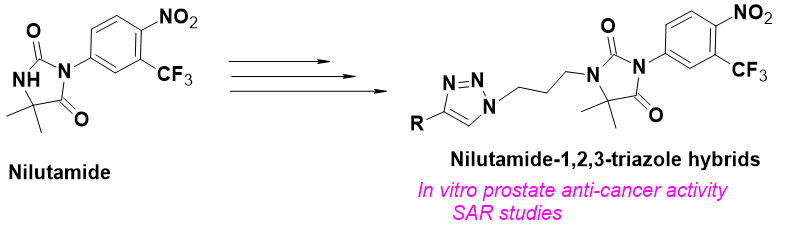
Design and synthesis of new Nilutamide-1,2,3-triazole derivatives as in vitro Anticancer agents
Keywords:
Anticancer, etoposide, triazole, Nilutamide, Anti-cancer, Tyrosine kinase, Prostate cancer, EGFR inhibitory activity, heterocyclic compoundsAbstract
The synthesis of novel 1,2,3-triazoles of Nilutamide (4a–4n) via Cu(I)-promoted 1,3-dipolar cycloaddition reaction between several terminal alkynes and 1-(3-azidopropyl)-5,5-dimethyl-3-(4-nitro-3-(trifluoromethyl)phenyl)imidazolidine-2,4-dione have been reported herein. In vitro anticancer activity studies of these synthesized compounds over two human prostate cell lines PC3 and DU-145 revealed that the compounds 4c, 4f and 4n exhibit slightly greater activity against two cell lines than the standard etoposide. Predominantly, the compound 4f displayed excellent activity over PC3 and DU-145 having IC50 values of 1.84and 1.34 μM respectively. The three most potent compounds 4c, 4f and 4n were also investigated for their inhibitory potential against tyrosine kinase EGFR and found that compound 4f showed superior activity than the standard erlotinib, while remaining two compounds 4c and 4n showed comparable activity with the standard.
URN:NBN:sciencein.cbl.2022.v9.405



