Synthesis and biological evaluation of Trifluoromethoxyphenyl Indole Carboxamide analogs, ADME and toxicity prediction
DOI:
https://doi.org/10.62110/sciencein.cbl.2024.v11.660Keywords:
Anticancer, Molecular docking study, Leukemia, Indole Carboxamide, heterocyclic drugsAbstract
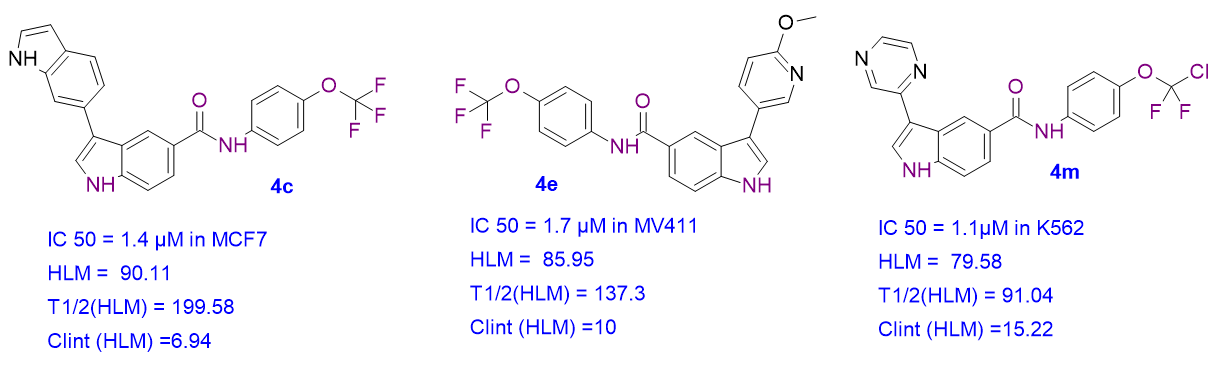
We report synthesis, ADME profile, and biological evaluation of new analogues as effective Anticancer Agents. Trifluoromethoxyphenyl indole-5-carboxamide analogues (4a-4m) were developed as a class of strong inhibitors of BCR-ABL1 kinase. The compounds (4c, 4e, and 4m) showed good anticancer activity in cancer cell lines such as MCF7, MV411 and K562 with IC50 values of 1.4 µM, 1.7 µM, and 1.1 µM, respectively. In human liver microsomes, these substances likewise displayed a favorable ADME profile, good solubility, and minimal clearance. In an oncology program these analogues offer a promising beginning for the development of BCR-ABL1 kinase inhibitors.
URN:NBN:sciencein.cbl.2024.v11.660







