Strain-promoted Azide-Alkyne cycloaddition: Cyclooctyne's potential in revolutionizing therapeutic and diagnostic applications through bio-orthogonal reactions
DOI:
https://doi.org/10.62110/sciencein.jmc.2025.1219Keywords:
Bioorthogonal Chemistry, Cyclooctyne, Florescent, Molecular Imaging, SPAAC, Azide-Alkyne Cycloaddition, Strained molecules, Click ChemistryAbstract
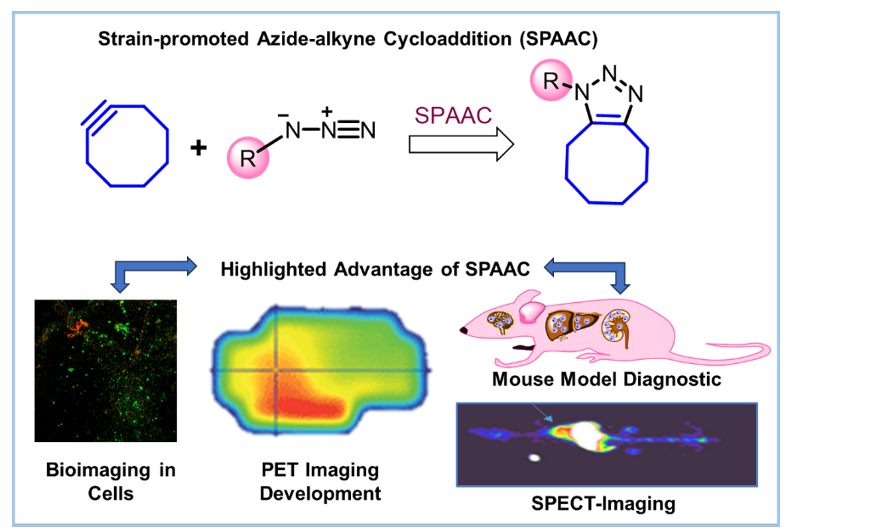
Strain-promoted azide–alkyne cycloaddition (SPAAC) with cyclooctyne derivatives has become a valuable tool in bio-orthogonal chemistry, enabling highly selective and efficient bioconjugation without the need for toxic copper catalysts. The intrinsic ring strain in cyclooctynes (~18 kcal mol⁻¹) drives rapid triazole formation under physiological conditions, making these reactions well suited for in vivo applications such as targeted drug delivery, molecular imaging, and real time biomolecule tracking. This review provides a detailed, well-suited, and up-to-date overview of cyclooctyne chemistry, encompassing structural evolution, mechanistic understanding, and diverse biomedical applications. Key advances of the last decade are highlighted, including innovative probe designs, stability-enhancing modifications, and comparative analyses of cyclooctyne analogues such as TCO, DIBO, and BCN. In contrast to earlier reviews, this article integrates both experimental and computational perspectives, critically evaluates recent translational progress, and directly compares functional performance across different strained alkyne systems. Furthermore, recent advancements in the development of cyclooctyne-based fluorescent and radiolabeled probes have opened up new avenues for the real time monitoring of intracellular events and disease progression. However, due to instability and nonspecificity under biological conditions, the therapeutic potential of these compounds remains limited, requiring further studies for clinical optimization. By combining breadth of coverage with in-depth mechanistic discussion, it provides a consolidated framework for researchers aiming to design next-generation cyclooctyne-based systems with improved selectivity, bioavailability, and clinical potential.
Downloads
Published
Issue
Section
URN
License
Copyright (c) 2025 Meenakshi Bansal, Reena Yadav, Pooja Kumari, Rajender Singh Malik, Sumit Kumar

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
