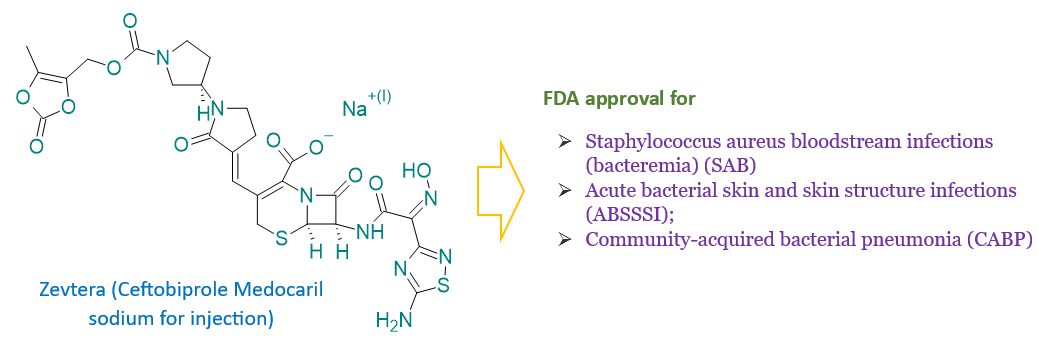
Ceftobiprole medocaril (Zevtera) – FDA approved antibiotic for three infections including methicillin-resistant Staphylococcus aureus
DOI:
https://doi.org/10.62110/sciencein.jmc.2024.692Keywords:
Drug Resistance, Antibiotics, Heterocyclic drugs, MRSA, Zevtera, antibacterial drug resistance, Basilea, beta-lactamAbstract
The U.S. Food and Drug Administration (FDA) has approved Zevtera (ceftobiprole medocaril sodium for injection) for multiple therapeutic indications. Zevtera (a product of Basilea company) is now authorized for use in adults to treat Staphylococcus aureus bloodstream infections (bacteremia), including cases of right-sided infective endocarditis. Additionally, it is approved for adults with acute bacterial skin and skin structure infections (ABSSSI). Further, Zevtera is also approved for treating community-acquired bacterial pneumonia (CABP) in pediatric patients aged three months to less than 18 years. This broad-spectrum antibiotic offers a significant advancement in managing severe bacterial infections across different age groups, enhancing treatment options for both healthcare providers and patients.
URN:NBN:sciencein.jmc.2024.692
Downloads
Published
Issue
Section
URN
License
Copyright (c) 2024 Liya Lee, Yi Zhang, Jyoti Singh

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
