Effect of size on Debye temperature, melting entropy and enthalpy of nanomaterials
Keywords:
Thermodynamic properties, Nanomaterials, Debye temperatureAbstract
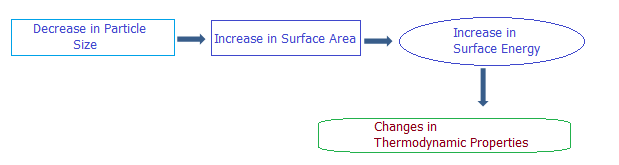
The energy associated with the free surface atoms will be different from the atoms of the bulk. The excess energy associated with the surface atom is called the free surface energy. In bulk materials, such free energy is neglected because it is associated with only few layers of atoms near the surface and the ratio of the volume occupied by the surface atoms and the total volume of the material is extremely small. However for the nanomaterials, the surface to volume ratio is significant. We report a theoretical model, free of adjustable parameters, the shape and size dependent Debye temperature, Melting entropy and Enthalpy of Au, Ag and In nanomaterials. We adopt the top down approach using classical thermodynamics by considering Lindemann’s criterion to study the size and shape effect. The results obtained are compared with the available experimental data.

