Synthesis, DNA photocleavage, molecular docking and anticancer studies of 2-methyl-1,2,3,4-tetrahydroquinolines
Abstract
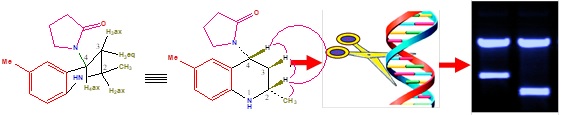
2-Methyl-1,2,3,4-tetrahydroquinolin-4-yl)pyrrolidin-2-ones (3a?g) were synthesized by one pot multicomponent aza Diels-alder reaction between N-arylimines with two molecules of N-vinyl-2-pyrrolidinone in presence of Sm(III)nitrate as catalyst in acetonitrile solvent at room temperature stirring. The photocleavage studies with 2-methyl-1,2,3,4-tetrahydroquinolin-4-yl)pyrrolidin-2-ones (3a?g) revealed that almost all derivatives exhibited effective photocleavage of pUC?19 DNA at 365 nm, The The anticancer activities of newly synthesized compounds (3a?g) were more potent than doxorubicin on MCF?7 cells. The docking of PBR receptor (1EQ1) protein with newly synthesized THQ’s (3a-g) exhibited well established bonds with one or more amino acids in the receptor active pocket.



