Design, synthesis and evaluation of Coumarin-Phenylthiazole conjugates as cholinesterase inhibitors
Abstract
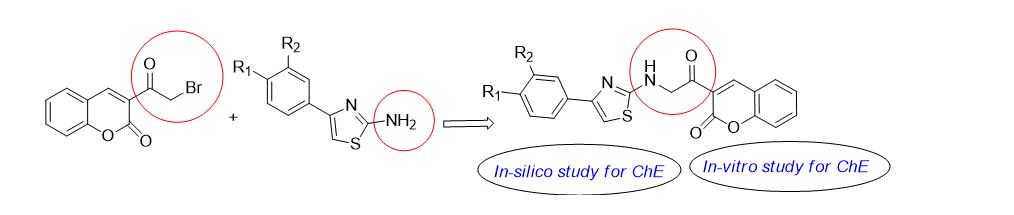
In this paper, we report the design, synthesis, in-silico, and in-vitro evaluations of a series of coumarin-phenylthiazole conjugates to inhibit cholinesterase enzymes. The coumarin and phenylthiazole derivatives have been synthesized separately, and further combined through covalent amine bond linkage. The synthesized compounds showed more inhibition towards BuChE than AChE, where 4-(3-bromophenyl)-1,3-thiazol-2-amine (7i) exhibited the strongest inhibition against BuChE with an IC50 value of 3.54 ?M. For the conjugates, 3-{2-[4-(3-nitrophenyl)thiazol-2-ylamino]acetyl}chromen-2-one (8j) exhibited strongest inhibition with an IC50 value of 46.47 ?M. The better inhibition activities towards BuChE are also shown by 3-bromo and 2-fluoro derivatives. It was also observed that the substitution at 3-position, on phenylthiazole moiety produced better results against BuChE than 4-substituted counterparts.



