A comparative study of isothermal nucleic acid amplification methods for SARS-CoV-2 detection at point-of-care
Keywords:
Covid-19, SARS-CoV-2, Corona virus, Detection, Diagnosis, RT-PCR, nucleic acid amplification test, colorimetric, direct diagnosisAbstract
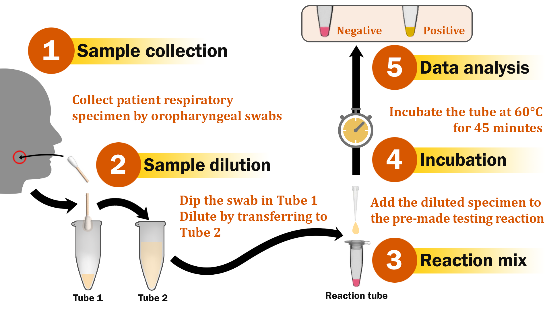
COVID-19, caused by the novel coronavirus SARS-CoV-2, has put most of the world under lockdown. Despite approved vaccines, COVID-19 cases, hospitalizations, and deaths have remained on the rise. Rapid diagnosis and necessary public health measures are still key parts to contain the pandemic. Here, the colorimetric isothermal nucleic acid amplification tests (iNAATs) for SARS-CoV-2 detection based on loop-mediated isothermal amplification (LAMP), cross-priming amplification (CPA), and polymerase spiral reaction (PSR) were designed and compared in performance for the first time. The findings showed that, for the detection of SARS-CoV-2 genomic-RNA, LAMP outperformed both CPA and PSR, exhibiting the limit of detection (LOD) of roughly 43.14 copies/reaction. The results can be read with the naked eye within 45 minutes, without cross-reactivity to closely related coronaviruses. The direct detection of SARS-CoV-2 RNA in simulated specimens by iNAATs was also successful. Additionally, the lyophilized reagents for LAMP reactions maintained the sensitivity and LOD of the liquid assays. The colorimetric LAMP assay was validated using clinical samples, showing 98.1% sensitivity and 100% specificity upon using extracted samples and 82.4% sensitivity and 86.2% specificity upon using unextracted specimens. The results indicate that the direct colorimetric LAMP assay developed is highly suitable for detecting SARS-CoV-2 at point-of-care.



