Design, synthesis, ADME prediction and anti-hyperglycemic evaluation of new alkoxyimino-substituted phenyl carboxylic acids as potent alpha-glucosidase inhibitors
Abstract
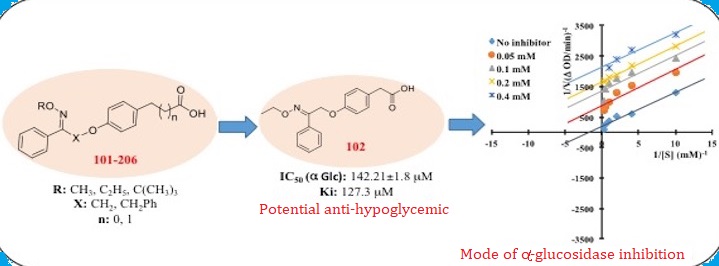
In an attempt to further explore the role of substituted carboxylic acid derivatives as antidiabetic agent, a series of alkoxyimino-substituted carboxylic acid derivatives (101-206) were designed, synthesized and evaluated for their inhibitory potential against a-amylase and a-glucosidase enzyme. Among all the tested compounds, 102 & 105 has displayed the most potent activity against a-glucosidase with the IC50 of 142.21±1.8 mM and 182.83±2.43 mM respectively, as compared to the standard drug acarbose (136.89±1.67 mM). Based on the inhibition percentage, the inhibition activity of 102 and 105 on a-glucosidase had higher potential than a-amylase. The mode of binding interactions between the a-glucosidase enzyme and the compound 102 was established to be uncompetitive using kinetic analysis. The predicted drug-likeness properties (Lipinski parameters and in silico ADME properties) of the compounds revealed their suitability as potential drug candidate.



